Introducción de Puntos de fusión del nailon 6 y el nailon 66
El nailon, un polímero sintético versátil, se utiliza ampliamente en numerosas industrias gracias a su resistencia, durabilidad y flexibilidad. Entre los distintos tipos de nailon, destacan el nailon 6 y el nailon 66. Aunque comparten muchas similitudes, sus puntos de fusión difieren significativamente. Aunque comparten muchas similitudes, sus puntos de fusión difieren significativamente. Este artículo profundiza en los puntos de fusión del nailon 6 y el nailon 66, explicando los factores que contribuyen a estas diferencias.
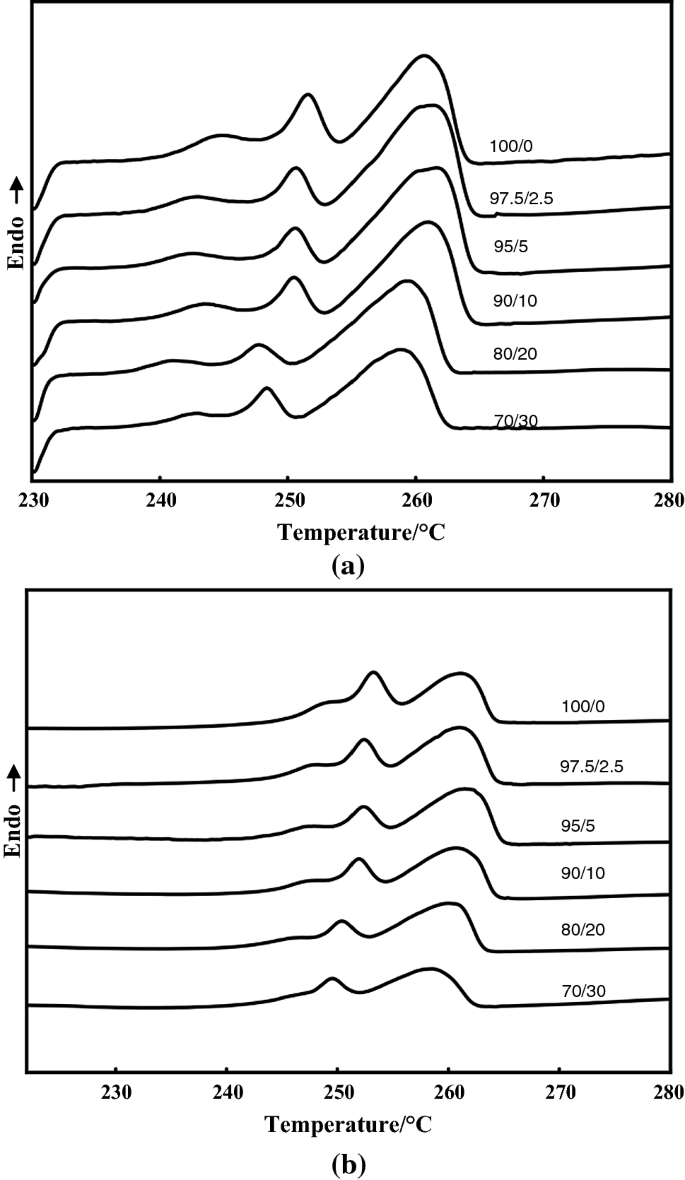
Punto de fusión de Nylon 6
Composición química y síntesis
El nailon 6, también conocido como policaprolactama (PCL), se produce mediante la polimerización de apertura en anillo de la caprolactama. Su punto de fusión oscila entre 215-225°C (420-437°F). Este punto de fusión relativamente bajo hace que el nailon 6 sea más fácil de procesar y moldear, lo que lo convierte en una opción popular para textiles, alfombras y piezas de automoción.
Punto de fusión de Nylon 66
Composición química y síntesis
El nailon 66, o polihexametileno adipamida (PA66), se sintetiza mediante la polimerización por condensación del ácido adípico y la hexametilendiamina. Su punto de fusión es más alto, en torno a 265-270°C (510-518°F). Aunque es más difícil de procesar debido a este punto de fusión más alto, el nailon 66 presenta propiedades mecánicas superiores, como una mayor resistencia, rigidez y resistencia química.
Factores que influyen en los puntos de fusión del nailon 6 y el nailon 66
- Estructura química
La razón principal de la diferencia en los puntos de fusión radica en las estructuras químicas de los polímeros. El nailon 6 consta de una unidad repetitiva de seis átomos de carbono, mientras que el nailon 66 incluye una unidad repetitiva de seis átomos de carbono y un grupo diamino adicional de seis carbonos. Este grupo de diamina en el Nylon 66 aumenta las fuerzas intermoleculares entre las cadenas del polímero, lo que se traduce en un punto de fusión más elevado.
- Cristalinidad
La cristalinidad también desempeña un papel importante. El nailon 6 tiene un menor grado de cristalinidad que el nailon 66, lo que contribuye a su menor punto de fusión. Esta menor cristalinidad permite que el nailon 6 fluya más fácilmente durante el procesado. Por el contrario, la mayor cristalinidad del Nylon 66 lo hace más rígido y difícil de procesar, contribuyendo así a su mayor punto de fusión.
- Condiciones de procesamiento
Las condiciones de procesamiento de estos nylons difieren debido a sus puntos de fusión. El nailon 6 se procesa a temperaturas más bajas, lo que facilita su manipulación y reduce el consumo de energía. Sin embargo, esto también limita la resistencia y rigidez máximas alcanzables de los productos de nailon 6. El Nylon 66, por su parte, requiere temperaturas de procesado más elevadas, lo que da lugar a productos con mayor resistencia y rigidez, pero también requiere un mayor consumo de energía y plantea más problemas de procesado debido a su mayor viscosidad.
Conclusión de MPuntos de fusión del nailon 6 y el nailon 66
En conclusión, los distintos puntos de fusión del nailon 6 y el nailon 66 se deben a diferencias en sus estructuras químicas, cristalinidades y condiciones de procesado. El nailon 6, con su punto de fusión más bajo, es más fácil de procesar pero ofrece propiedades mecánicas inferiores. Por el contrario, el Nylon 66, con un punto de fusión más alto, ofrece una resistencia y rigidez superiores, pero es más difícil de procesar. Comprender estas diferencias es esencial para seleccionar el tipo de nailon adecuado para aplicaciones y requisitos de procesamiento específicos.
FAQ: Puntos de fusión del nailon 6 y el nailon 66
1. ¿Qué son el nailon 6 y el nailon 66?
- Nylon 6: También conocido como policaprolactama (PCL), sintetizado por polimerización de apertura en anillo de la caprolactama.
- Nylon 66: También conocida como polihexametileno adipamida (PA66), sintetizada por la polimerización por condensación del ácido adípico y la hexametilendiamina.
2. ¿Cuál es el punto de fusión del nailon 6?
- El nailon 6 tiene un punto de fusión de aproximadamente 215-225°C (420-437°F).
3. ¿Cuál es el punto de fusión del nailon 66?
- El nailon 66 tiene un punto de fusión de aproximadamente 265-270°C (510-518°F).
4. ¿Por qué el nailon 6 tiene un punto de fusión más bajo que el nailon 66?
- Estructura química: El nailon 6 tiene una estructura más simple con seis átomos de carbono en su unidad repetitiva, mientras que el nailon 66 tiene un grupo diamino adicional de seis carbonos, lo que aumenta las fuerzas intermoleculares y el punto de fusión.
- Cristalinidad: El nailon 6 tiene menor cristalinidad, lo que facilita su procesamiento, pero con un punto de fusión inferior en comparación con la mayor cristalinidad y rigidez del nailon 66.
5. ¿En qué difieren las condiciones de transformación del nailon 6 y del nailon 66?
- Nylon 6: Se procesa a temperaturas más bajas debido a su menor punto de fusión, lo que facilita su manipulación y reduce el consumo de energía, pero limita sus propiedades mecánicas.
- Nylon 66: Requiere temperaturas de procesamiento más elevadas, lo que permite obtener productos más resistentes y rígidos, pero con un mayor consumo de energía y un procesamiento más difícil debido al aumento de la viscosidad.
6. ¿Qué aplicaciones tienen el nailon 6 y el nailon 66?
- Nylon 6: Comúnmente utilizado en textiles, alfombras y piezas de automóviles debido a su facilidad de procesamiento.
- Nylon 66: Preferido para aplicaciones que requieren una mayor resistencia y resistencia química, como en plásticos de ingeniería y componentes industriales.
7. ¿Cómo ayuda a seleccionar el material adecuado conocer los puntos de fusión del nailon 6 y el nailon 66?
- Conocer los puntos de fusión del nailon 6 y el nailon 66 y sus propiedades asociadas ayuda a elegir el tipo de nailon adecuado para aplicaciones y condiciones de procesado específicas, equilibrando factores como la facilidad de procesado, la resistencia mecánica y el consumo de energía.
