Einführung
Nylon ist heute eines der vielseitigsten und am häufigsten verwendeten synthetischen Polymere der Welt. Seine bemerkenswerte Festigkeit, Flexibilität und Haltbarkeit machen es zu einem unverzichtbaren Material für verschiedene Branchen, von der Bekleidung bis hin zu industriellen Anwendungen. Hinter seiner Herstellung steht jedoch ein komplexer Prozess, der als Nylonpolymerisation bekannt ist. Für jeden, der sich für Materialwissenschaften oder industrielle Fertigung interessiert, ist es wichtig zu verstehen, wie Nylon synthetisiert wird, welche Eigenschaften es hat und welche Anwendungen es bietet.
Dieser Artikel befasst sich ausführlich mit dem Polymerisationsprozess von Nylon, untersucht seine verschiedenen Arten und Verwendungszwecke und geht auf einige häufig gestellte Fragen zu seiner Herstellung und seinem Recycling ein. Egal, ob Sie Student, Ingenieur oder Fachmann in der Industrie sind, dieser Leitfaden wird Ihnen wertvolle Einblicke in eines der wichtigsten Materialien in der modernen Fertigung geben.
Die Polymerisation von Nylon verstehen
Definition von Polymerisation
Polymerisation ist ein chemischer Prozess, bei dem kleine Moleküle, so genannte Monomere, chemisch miteinander verbunden werden, um lange Ketten oder Polymere zu bilden. Das resultierende Polymer hat Eigenschaften, die sich von denen der einzelnen Monomere unterscheiden. Im Falle von Nylon beinhaltet die Polymerisation die Verknüpfung von Monomeren, um eine lange, flexible und dauerhafte Kette aus sich wiederholenden Einheiten zu bilden.
Die Polymerisation von Nylon erfolgt durch zwei Hauptmethoden: Kondensationspolymerisation und Additionspolymerisation. Beide Methoden sind bei der Herstellung verschiedener Nylonarten weit verbreitet, aber die Kondensationspolymerisation ist das häufigste Verfahren zur Herstellung von Nylon.
Die Rolle der Monomere bei der Bildung von Nylon
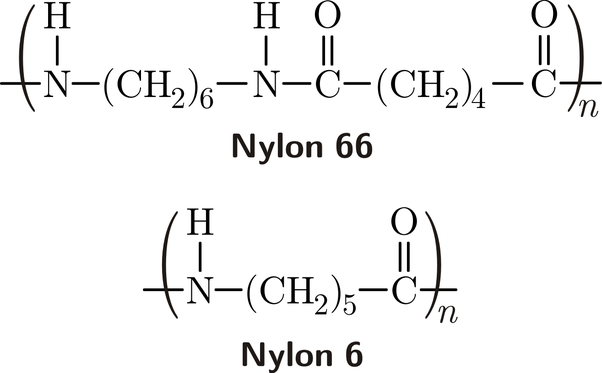
Bei der Nylonpolymerisation sind die Monomere in der Regel Diamine und Dicarbonsäuren. Die Diamin- und Dicarbonsäuremoleküle enthalten jeweils funktionelle Gruppen, die reaktiv sind, so dass sie sich während der Polymerisation miteinander verbinden können. Das am häufigsten verwendete Diamin ist Hexamethylendiamin, während die verwendete Dicarbonsäure Adipinsäure ist. Wenn diese Monomere polymerisiert werden, bilden sie eine sich wiederholende Einheit, die als Nylon-6,6 bekannt ist.
Durch die Kombination dieser beiden Monomere entsteht eine starke, flexible Polymerkette, die charakteristisch für die beeindruckenden mechanischen Eigenschaften von Nylon ist.
Verschiedene Arten von Nylon und ihre Eigenschaften
Nylon gibt es in mehreren Varianten, die jeweils für bestimmte Anwendungen geeignet sind. Die gängigsten Typen sind:
Nylon 66: Dies ist die am häufigsten verwendete Form von Nylon. Es ist bekannt für seine Festigkeit, Haltbarkeit und seinen hohen Schmelzpunkt, wodurch es sich ideal für Anwendungen wie Textilien, Automobilteile und Industrieanlagen eignet.
Nylon 6: Das durch Polymerisation von Caprolactam hergestellte Nylon-6 ist etwas flexibler als Nylon-6,6 und wird häufig für Textilien, Seile und Fischernetze verwendet.
Nylon 12: Aufgrund der verbesserten chemischen Beständigkeit und der geringeren Wasseraufnahme wird Nylon-12 häufig für Automobilteile, Kraftstoffleitungen und medizinische Geräte verwendet.
Jeder Nylontyp hat spezifische Eigenschaften, die ihn für verschiedene Anwendungen geeignet machen, von Textilien bis hin zu technischen Materialien.
Der Prozess der Polymerisation von Nylon
Schritt-für-Schritt-Erläuterung des Nylon-Polymerisationsprozesses
Die Polymerisation von Nylon erfolgt in der Regel durch eine schrittweise Kondensationsreaktion, bei der die Monomere zu langen Ketten verbunden werden. Hier ist eine schrittweise Aufschlüsselung des Prozesses:
Herstellung von Monomeren: In einem ersten Schritt werden die Monomere, wie Hexamethylendiamin und Adipinsäure, unter kontrollierten Bedingungen hergestellt.
Polymerisationsreaktion: Die Monomere werden in Anwesenheit eines Katalysators erhitzt. Bei der Reaktion werden Wassermoleküle freigesetzt, wenn sich die Diamin- und Dicarbonsäuremoleküle miteinander verbinden.
Bildung von Polymerketten: Im weiteren Verlauf der Reaktion verbinden sich die Monomere und bilden lange Polymerketten. Die Polymerisationsreaktion kann mehrere Stunden dauern, um sicherzustellen, dass die Ketten ausreichend lang sind.
Abkühlung und Erstarrung: Nachdem sich die Polymerketten gebildet haben, wird die Mischung abgekühlt und verfestigt. Das Ergebnis ist ein festes Nylonmaterial, das zu Fasern, Platten oder anderen Formen weiterverarbeitet werden kann.
Faktoren, die die Polymerisationsreaktion beeinflussen
Mehrere Faktoren beeinflussen die Effizienz und Qualität des Nylonpolymerisationsprozesses:
Temperatur: Die Temperatur, bei der die Polymerisation stattfindet, beeinflusst die Reaktionsgeschwindigkeit und das Molekulargewicht des entstehenden Nylons. Hohe Temperaturen beschleunigen die Polymerisation, aber übermäßige Hitze kann zu einem Abbau führen.
Katalysatoren: Der Einsatz von Katalysatoren beschleunigt die Reaktion, indem er die Aktivierungsenergie senkt, so dass die Monomere leichter reagieren und Polymerketten bilden können.
Monomer-Konzentration: Die Konzentration von Diamin und Dicarbonsäure beeinflusst die Reaktionsgeschwindigkeit und die Eigenschaften des Endprodukts.
Reaktionszeit: Die Dauer der Reaktionszeit kann das Molekulargewicht des Polymers beeinflussen. Längere Reaktionszeiten führen in der Regel zu Nylon mit höherem Molekulargewicht und besseren mechanischen Eigenschaften.
Herausforderungen und Überlegungen bei der Nylonproduktion
Obwohl die Nylonpolymerisation ein bewährtes Verfahren ist, gibt es bei seiner Herstellung mehrere Herausforderungen. Eines der Hauptprobleme ist die Freisetzung von Wasser während der Kondensationspolymerisation. Dieses Nebenprodukt kann die Qualität des Nylons beeinträchtigen, wenn es nicht sorgfältig behandelt wird. Um das gewünschte Molekulargewicht und die gewünschte Kettenlänge des Polymers zu erreichen, müssen außerdem die Reaktionsbedingungen genau kontrolliert werden.
Ein weiterer Aspekt ist der Energieverbrauch bei der Nylonherstellung. Die für die Polymerisation erforderlichen hohen Temperaturen können energieintensiv sein, was Bedenken hinsichtlich der Umweltauswirkungen der Nylonherstellung aufkommen lässt.
Anwendungen der Nylonpolymerisation
Häufige Verwendungen von Nylon in verschiedenen Branchen
Nylon ist ein äußerst vielseitiges Material, das aufgrund seines Polymerisationsverfahrens in einer Vielzahl von Anwendungen eingesetzt werden kann. Einige der häufigsten Anwendungen sind:
Textilien: Nylon wird in der Modeindustrie in großem Umfang zur Herstellung von haltbaren, leichten Stoffen verwendet, z. B. für Kleidung, Strümpfe und Polstermöbel.
Automobilindustrie: Nylon wird aufgrund seiner Festigkeit und Verschleißbeständigkeit für die Herstellung verschiedener Automobilteile wie Getriebe, Motorteile und Kraftstoffleitungen verwendet.
Industrielle Ausrüstung: Die Abrieb- und Stoßfestigkeit von Nylon macht es zu einer idealen Wahl für Lager, Buchsen und Förderbänder.
Medizinische Geräte: Nylon wird aufgrund seiner Biokompatibilität und Haltbarkeit für medizinische Anwendungen wie chirurgisches Nahtmaterial, medizinische Schläuche und Prothesen verwendet.
Vorteile von Nylon gegenüber anderen Materialien
Nylon bietet mehrere Vorteile gegenüber anderen Materialien wie Metallen und Naturfasern:
Stärke und Langlebigkeit: Nylon ist für seine überragende Zugfestigkeit bekannt und eignet sich daher ideal für stark beanspruchte Anwendungen.
Leichtgewicht: Trotz seiner Stärke ist Nylon leicht und eignet sich daher perfekt für Anwendungen, bei denen das Gewicht eine Rolle spielt, wie z. B. in der Luft- und Raumfahrt und in der Automobilindustrie.
Chemische Beständigkeit: Nylon ist gegen eine Vielzahl von Chemikalien, Ölen und Lösungsmitteln beständig und eignet sich daher für den Einsatz in rauen Umgebungen.
Zukunftsperspektiven der Nylon-Polymerisationstechnologie
Da die Nachfrage nach nachhaltigen Materialien steigt, suchen Forscher nach Möglichkeiten, den Polymerisationsprozess von Nylon zu verbessern. Innovationen wie biobasierte Nylons, die aus erneuerbaren Ressourcen wie Rizinusbohnen hergestellt werden, sind auf dem Vormarsch. Diese Alternativen zielen darauf ab, die Umweltauswirkungen der Nylonproduktion zu verringern und gleichzeitig die gewünschten Eigenschaften beizubehalten.
Darüber hinaus verbessern Fortschritte bei den Recyclingtechnologien die Wiederverwendbarkeit von Nylonprodukten und tragen so zu einer stärker kreislauforientierten Wirtschaft bei.
Häufig gestellte Fragen
1. Was ist der Unterschied zwischen Kondensations- und Additionspolymerisation?
Bei der Kondensationspolymerisation wird bei der Bindung der Monomere ein kleines Molekül, in der Regel Wasser, abgespalten. Im Gegensatz dazu werden bei der Additionspolymerisation keine Nebenprodukte freigesetzt. Beide Verfahren werden zur Herstellung verschiedener Nylontypen verwendet, wobei die Kondensationspolymerisation am häufigsten zur Herstellung von Nylon-6,6 eingesetzt wird.
2. Wie unterscheidet sich die Polymerisation von Nylon von anderen Polymerisationsverfahren?
Die Nylonpolymerisation ist eine spezielle Art der Kondensationspolymerisation, die die Kombination von Diaminen und Dicarbonsäuren erfordert. Im Gegensatz zu anderen Polymerisationsverfahren, wie z. B. für Polyethylen, führt die Nylonpolymerisation zu einem Material mit hervorragenden mechanischen Eigenschaften, chemischer Beständigkeit und Hitzebeständigkeit.
3. Kann Nylon nach der Polymerisation recycelt werden?
Ja, Nylon kann durch verschiedene Verfahren recycelt werden, einschließlich des mechanischen Recyclings, bei dem alte Nylonprodukte zerlegt und zu neuen Materialien verarbeitet werden. Die Effizienz des Recyclings von Nylon hängt jedoch von der spezifischen Art des Nylons und der Qualität des Recyclingverfahrens ab.
Schlussfolgerung
Die Polymerisation von Nylon ist ein entscheidender Prozess, der zu einem der am häufigsten verwendeten und vielseitigsten Materialien in der modernen Industrie führt. Von den anfänglichen Monomeren bis zum Endprodukt ist der Prozess der Nylonherstellung komplex und erfordert eine sorgfältige Kontrolle verschiedener Faktoren. Die vielfältigen Anwendungen von Nylon - von Textilien bis hin zu Automobilteilen - zeigen, wie wichtig es in verschiedenen Sektoren ist. Im Zuge des technologischen Fortschritts sind weitere Innovationen bei der Herstellung und dem Recycling von Nylon zu erwarten, so dass dieses Material auch in den kommenden Jahren eine wichtige Rolle in der Fertigungswelt spielen wird.
